LLNL researchers predict the movement of radioactive isotopes above and below Earth’s surface, fostering long-term environmental stewardship.
Actinide-related environmental research
Plutonium is the most abundant and chemically complex anthropogenic actinide. Since the dawn of the nuclear era, the inventory of plutonium released in our environment has increased dramatically as a result of nuclear weapons production and testing, nuclear accidents, and inadequate waste management practices. In addition to being highly toxic and long-lived, plutonium has been shown to migrate in the environment—in soil, as well as in rivers, streams, and groundwater.
For decades, LLNL scientists have studied the environmental behavior of radioactive elements such as plutonium and uranium. The research is driven by the Laboratory’s historic role assessing the nation’s nuclear stockpile, ensuring the safe storage of nuclear waste, and evaluating the fate and transport of radioactive isotopes in the environment.
This type of research provides decision makers with the scientific basis to support plans for remediation and long-term stewardship of sites where actinide contamination has occurred. It also provides scientific support to explore long-term storage of nuclear materials as part of nuclear waste repository science.
Understanding actinide transport through field observations and experiments
LLNL research includes lab-based experiments, field observations, and computational models aimed at understanding the behavior of actinides.
- Laboratory experiments provide quantitative data regarding the affinities and kinetics of actinide interactions with mineral surfaces, organic matter, and microbes.
- Field observations provide direct evidence of the behavior of actinides in the environment at sites that represent diverse environmental challenges.
- Predictive models regarding the migration behavior of actinides in the environment are calibrated to findings from field and laboratory studies.
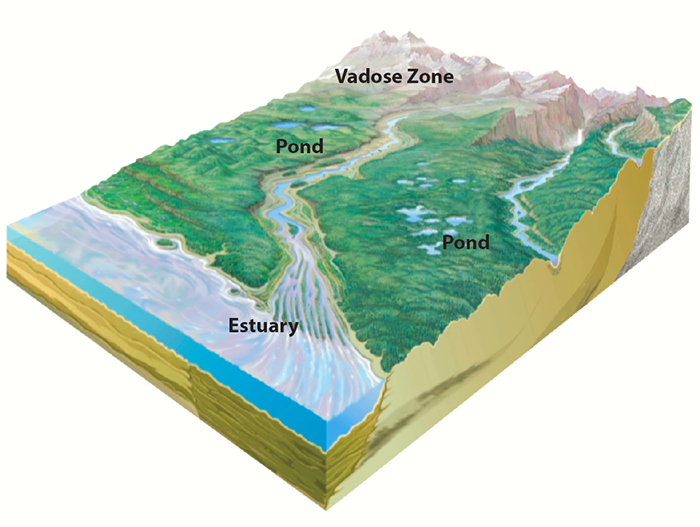
Our field observations take place at sites representing diverse ecosystems, enabling us to study the connection between specific environmental conditions and actinide mobility. Our field sites include:
- Water drainage ponds at a nuclear testing site.
- Reactor cooling ponds linked to a river system.
- An estuary located near a processing facility, where freshwater meets seawater.
- A contaminated vadose zone, located between the land surface and an aquifier, where flow rates and chemical reactions determine how rapidly contaminants enter the groundwater.
- Rock formations that may serve as potential nuclear waste repositories.
Isotope hydrology
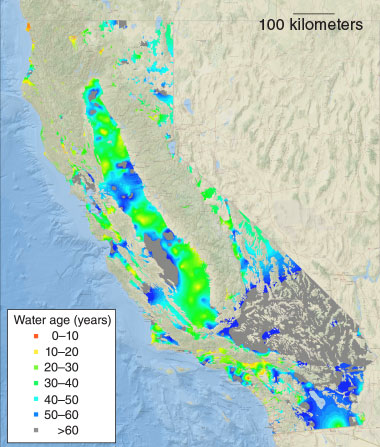
Environmental radiochemistry research at LLNL also includes a wide range of isotope geochemistry research, such as studying the composition of the Earth’s mantle and developing new methods to study underground aquifers and other freshwater resources.
Using naturally occurring and anthropogenic isotopes, LLNL scientists use hydrology to trace the movement of water, which enables them to study how quickly it is replenished and whether it is at risk of contamination. For example, researchers can determine when groundwater was recharged from surface water that percolated down through the soil into the aquifer. Knowing the recharge time helps researchers determine the source of contaminants and the flow of groundwater. Researchers also use the isotopic composition of pervasive pollutants, such as nitrates, to determine the source of contamination. Learn more about LLNL’s isotope hydrology research.
Learn more about environmental radiochemistry research at LLNL
Tracking Plutonium through the Environment, Science & Technology Review magazine, March 2021
Investigating Water under Earth’s Surface, Science & Technology Review magazine, December 2017
Uncovering Dirty Secrets about Soil Carbon, Science & Technology Review magazine, April/May 2016
Protecting Aquifers to Secure Clean Water for California, Science & Technology Review magazine, March 2016
Plutonium Hitches a Ride on Subsurface Particles, Science & Technology Review magazine, October/November 2011