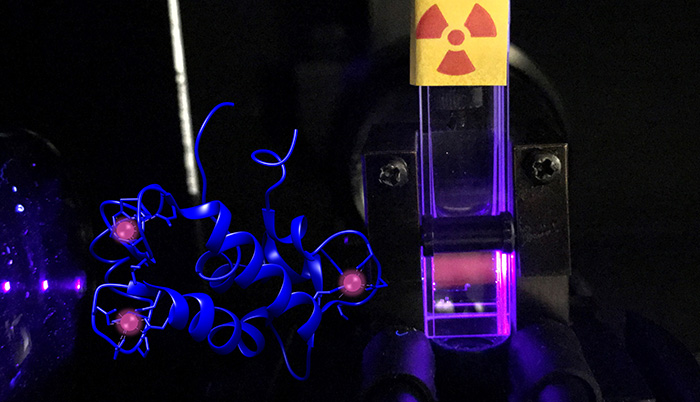
LLNL scientists advance fundamental knowledge in actinide chemistry—studying the unique properties of some of the heaviest elements on Earth.
Elements with exotic chemical properties
The most well-known actinides are uranium and plutonium, but the actinide series on the periodic table goes from actinium (Z = 89) to lawrencium (Z = 103). All of them are radioactive, and most emit highly energetic alpha particles, necessitating special research facilities and safety precautions, such as those found at LLNL.
Actinide research is particularly interesting because these elements have exotic chemical properties when compared to more common elements, such as iron and other natural metals. In addition to their radioactive nature, actinides exhibit a broad range of oxidation states and electronic structures. They can also form actinyl ions, a feature not seen in other elements. In addition, actinides have high coordination numbers, and relativistic effects start influencing their chemical behavior.
Actinides are the heaviest elements on Earth for which bulk chemistry experiments can be performed. As such, by studying the fundamental properties of actinides, scientists can better understand how chemical principles apply to this unique group of elements.
Mission-relevant actinide chemistry
Actinide chemistry is an integral part of several mission-relevant research areas at LLNL, including nuclear energy, nuclear physics, nuclear waste management, and nuclear medicine. This research also supports the Lab’s missions in space science and security and nuclear deterrence.
For example, using a broad variety of instrumentation, spectroscopic techniques, field observations, and computational models, our scientists study the interactions between actinides and natural molecules. They also explore how to detect and analyze actinide materials.
Other examples of LLNL’s actinide chemistry research include:
- New methods for purifying and studying actinium and other radionuclides used in nuclear medicine, including cancer therapies.
- The interplay between microbial activity and actinide chemistry.
- Identification and characterization of natural compounds (e.g., water soluble organic ligands, proteins, humic acid substances, and minerals) that are likely to interact with radionuclides.
- Biochemistry of f-block elements on the periodic table.
- Biogeochemical mechanisms that control plutonium mobility in soil and groundwater.
Learn more about actinide chemistry research at LLNL
Going beyond Mother Nature’s molecules to target radioactive metals, LLNL news, May 2022
Cancer therapies and nuclear material detection get a boost from newly discovered protein, LLNL news, October 2021
Nuclear waste interaction in the environment may be more complicated than once thought, LLNL news, September 2021
Using environmental microbes to remove uranium from groundwater, LLNL news, May 2021